Pancreatic carcinoma is the fourth leading cause of cancer death in Europe and is expected to rank second in cancer mortality by 2030 [1]. The only potentially curative treatment option is surgical resection, which still achieves a 5-year survival rate of only 10% [2]. The aggressive tumor biology has led to the introduction of new, more effective chemotherapeutic regimens in the last 10 years, both adjuvant and neoadjuvant, resulting in the establishment of multimodal treatment concepts.
Indication for Surgery
At the initiative of the German Society for General and Visceral Surgery (DGAV), evidence-based recommendations for the indication for surgery in pancreatic carcinoma were defined, whereby the indication should be made by a tumor board of experienced pancreatic surgeons in accordance with guidelines, taking into account individual patient characteristics [3]. According to the recommendations, which are based on the systematic analysis of 58 original articles and 10 guidelines, there is an indication for surgery in histologically proven pancreatic carcinoma as well as in high suspicion of a resectable pancreatic carcinoma [3, 4].
Resectability
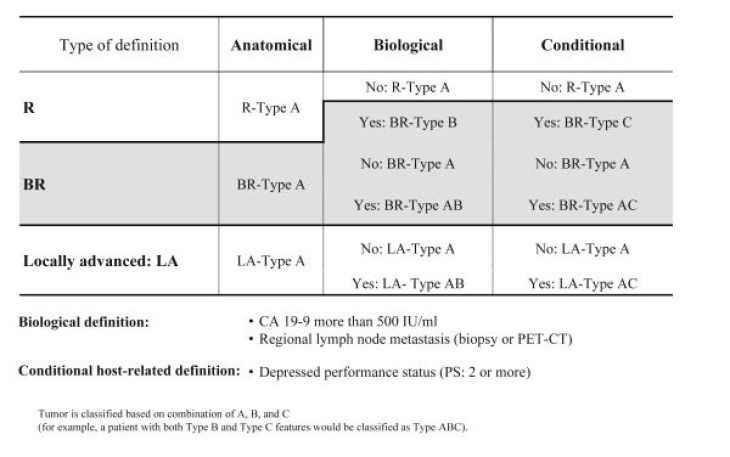
The greatest probability of survival exists with resection in healthy tissue, the R0 resection [5, 6]. In the current guidelines, the R0 classification is now divided into “R0 narrow” (≤ 1 mm) and “R0 wide” (> 1 mm), if the carcinoma approaches the resection margin less or more than one millimeter [7]. In addition to anatomical resectability (positional relationship between tumor and large visceral vessels), tumor biology and the patient's general condition have been considered decisive resectability criteria since 2017 and incorporated into the current S3 guidelines as the ABC consensus classification of resectability [8].
ABC Criteria of Resectability According to the International Association of Pancreatology (IAP) Consensus
(Click to enlarge)
Source: Isaji S et al (2018) International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology18(1):2–11.
To assess anatomical resectability, the S3 guidelines recommend a contrast-enhanced 2-phase computed tomography [7]. Based on the anatomical resectability criteria, a tumor can be classified as primarily resectable, borderline resectable, and non-resectable or locally advanced [7].
The assessment of biological resectability is most commonly done using the tumor marker CA 19-9. The threshold was defined as > 500 IU/ml, since above this value resectability is present in only less than 70% of cases and survival of less than 20 months is expected [8, 9].
As another criterion, the ECOG Performance Status is used as conditional resectability, whereby patients with a status ≥ 2 have a poor prognosis [8].
Mesopancreas
The mesopancreas, the connective tissue region around the large vessels of the pancreatic region, which is densely traversed by blood and lymph vessels as well as nerve plexuses, has been discussed for several years [10]. Meta-analyses suggest that total mesopancreatic resection enables better oncological results [11]. In pancreatic head resection, complete removal of the mesopancreatic tissue between the portal vein, hepatic artery, base of the celiac trunk, and superior mesenteric artery (triangle operation [12, 13]) is performed; in left pancreatic resections (body, tail carcinomas), radical antegrade modular pancreatosplenectomy (RAMPS [14]) is performed.
[RAMPS: Here, depending on the tumor extension, an anterior is distinguished from a posterior RAMPS procedure, in which resection is essentially more radical dorsally. In anterior RAMPS, resection includes the Gerota's fascia and perirenal fat on the left side. In contrast, posterior RAMPS additionally resects the left adrenal gland in addition to Gerota's fascia and perirenal fat.]
Vascular Resection
In venous resections in centers, morbidity and mortality are minimally increased and adequate overall survival is enabled [15, 16]. Thus, according to the current S3 guidelines, resection of the portal vein can be performed in tumor infiltration ≤ 180° or even in complex situations such as cavernous transformation with reconstruction [17]. Arterial resections, on the other hand, are very risky, often complex, and not infrequently require simultaneous venous reconstructions. Patients often do not benefit oncologically from the extensive procedures and not infrequently show worse survival data than patients without vascular resection [18]. Arterial resections should therefore be avoided outside of centers.
Unexpected arterial resections can be avoided by checking the tumor-free status of the superior mesenteric artery and celiac trunk through early exposure in a curatively intended pancreatic resection. The “artery-first” strategy helps to avoid frustrating procedures, enables better planning of vascular resections and reconstructions, and improves long-term survival for selected patients in centers with appropriate expertise [19].
Oligometastasis
The term oligometastasis appears for the first time in the current S3 guidelines and describes the presence of ≤ 3 metastases, which should only be resected within studies as part of a multimodal treatment concept [7]. There are no randomized studies yet, but resection of oligometastases seems to improve patient survival data compared to palliative chemotherapy, especially after neoadjuvant therapy [20 - 23]. In Germany, the HOLIPANC and METAPANC studies are currently addressing the topic [24].
Neoadjuvant Treatment Concepts
In patients with borderline resectable pancreatic carcinoma, the current guideline recommends preoperative chemotherapy or radiochemotherapy; in resectable carcinomas, it should not take place outside of studies [7]. The recommendations are based on the data of a meta-analysis as well as recently published study data [25, 26]. Since after neoadjuvant therapy, resectability in initially borderline resectable and locally advanced pancreatic carcinomas is difficult to assess morphologically, the guideline recommends surgical exploration in stable disease to assess secondary resectability [7, 27]. In assessing secondary resectability, the drop in CA 19-9 value can also help [28, 29].
Laparoscopic Techniques and Robotics in Pancreatic Carcinoma
Left pancreatic and pancreatic head resections must be considered differently. For left resections in laparoscopic technique, the randomized controlled LEOPARD study showed faster convalescence, lower blood loss, and no higher complication rate compared to the open technique [30]. In the combined analysis of the LEOPARD and LAPOPS study, the data were confirmed [31]. Long-term quality of life remains unchanged by the laparoscopic technique [32]. A meta-analysis of the existing data yielded comparable results for the R0 resection rate and the rate of adjuvant chemotherapy [33]. The median overall survival was the same at 28 and 31 months for laparoscopic and open left pancreatic resections [34].
For pancreatic head resections, the randomized and controlled LEOPARD-2 study published in 2019 showed higher mortality (90-day mortality 10%) in the laparoscopic group, which showed no advantages over the open group in terms of postoperative pain, convalescence, hospital stay, and quality of life [36]. A recent Chinese randomized study showed comparable mortality in laparoscopic pancreatic head resection with only a slight advantage of the laparoscopic technique [37].
Robotics has also become established in pancreatic surgery in the last 10 years. In addition to the technically simpler left resection, pancreatoduodenectomy is increasingly being performed. However, a long learning curve is required here [37] and a final evaluation regarding oncological results is not yet possible. Only observational studies are available on the use of robotics for malignant indications, which demonstrate feasibility and potential advantages of the minimally invasive technique [38, 39, 40]. According to international guidelines, a malignant indication is not a fundamental contraindication for robotics, but results from randomized controlled trials and thus high-quality results are expected in 3 to 5 years [41].
Centralization of Pancreatic Surgery
In high-volume centers for pancreatic surgery, postoperative mortality can be reduced and survival increased [42, 43, 44]. Against this background, following the decision of the Joint Federal Committee in Germany, the minimum volumes for complex pancreatic procedures will be increased from the current 10 to 20 resections per year starting in 2024.
Whipple Operation versus Pylorus-Preserving Pancreatoduodenectomy (PPPD)
Two surgical procedures are considered for the resection of pancreatic head and periampullary carcinomas: the classic resection according to Kausch-Whipple and pylorus-preserving pancreatoduodenectomy. The latter has the advantage of preserved physiological food passage and reduction of dumping syndromes, postoperative weight loss, and reflux [45-52].
The more recent studies [49, 51, 52] were able to show a lower transfusion rate and hospital stay in PPPD patients compared to the Whipple group. Postoperative morbidity did not differ significantly in both groups. The occurrence of delayed gastric emptying was comparable in both groups (Whipple 23% vs. PPPD 22%). Regarding surgical radicality, there was also no significant difference (R0-Whipple 82.6% vs. R0-PPPD 73.6%). Long-term follow-up showed comparable overall survival rates.