Abdominal Wall Hernias
Primary (umbilical hernias, epigastric hernias) and secondary (incisional hernias) abdominal wall hernias are among the most common surgical indications in general and visceral surgery. In 2018, 60,566 umbilical hernias, 49,387 incisional hernias, and 10,695 epigastric hernias were treated inpatient in Germany [1]. Despite the frequency of these procedures, the evidence-based data for specific therapeutic decisions in the guidelines is considered insufficient [2, 3, 4, 5, 6].
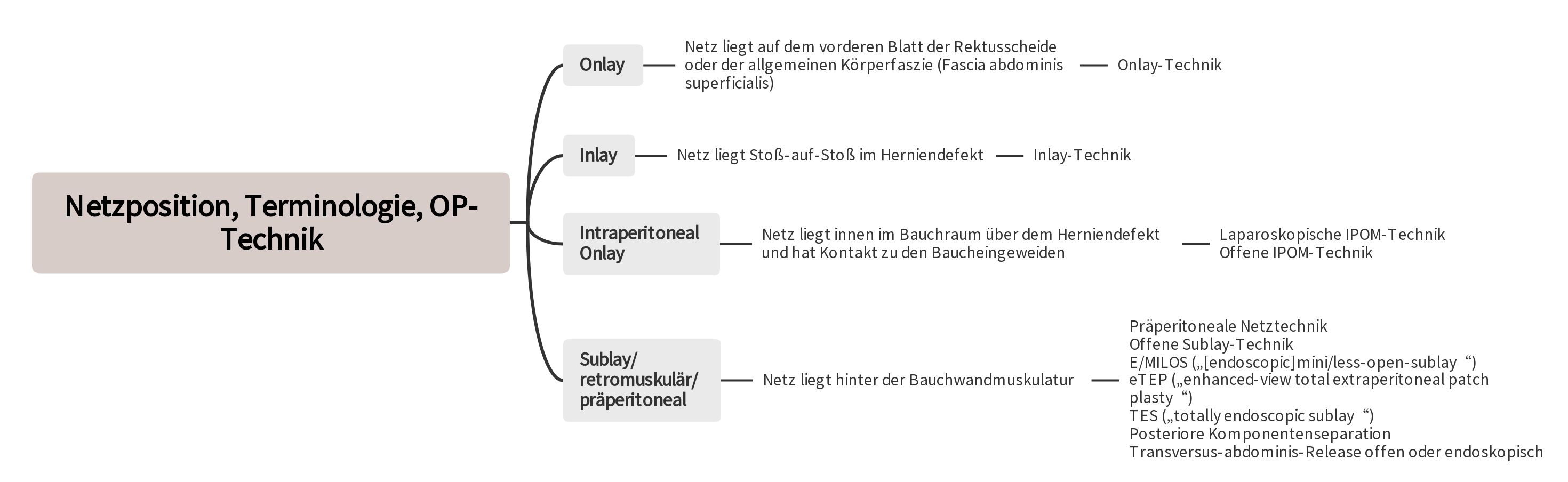
The use of mesh techniques represents the standard in the treatment of abdominal wall hernias [2, 3, 4, 5, 6]. In particular, the retromuscular positioning of the mesh behind the abdominal wall musculature and outside the abdominal cavity is preferred [2, 3, 4, 5, 6].
To reduce the high rate of wound complications in open surgeries and avoid potential risks of mesh contact with the abdominal viscera in the IPOM technique, various innovative techniques have been developed [3, 4, 6]. These new approaches are characterized by the placement of the mesh through small incisions or endoscopically into the sublay/retromuscular/preperitoneal layer.
Classification of Abdominal Wall Hernias
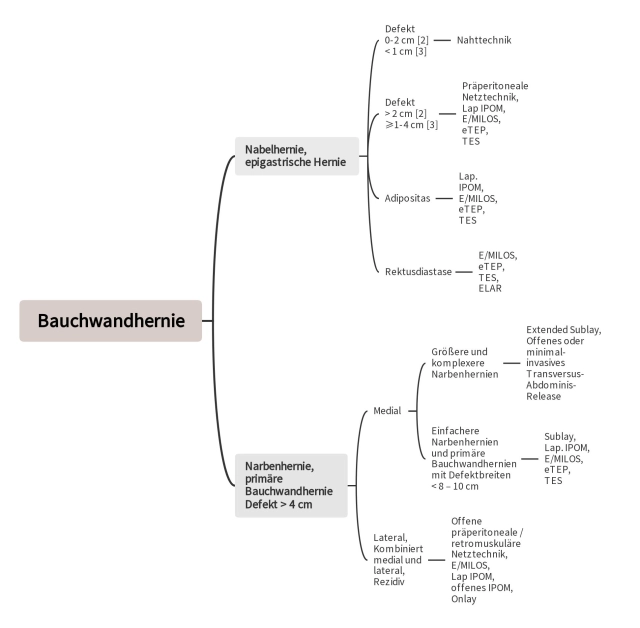
The European Hernia Society has developed a classification for primary and secondary abdominal wall hernias [7]. In terms of their defect diameter, primary abdominal wall hernias such as umbilical hernias and epigastric hernias are classified as small (< 2 cm), medium (≥ 2–4 cm), and large (> 4 cm). However, this simple classification is problematic for primary abdominal wall hernias in the midline, especially when a rectus diastasis is present. In these situations, it is advisable to determine and consider the width and length of the rectus diastasis [8].
The classification of secondary abdominal wall hernias (incisional hernias) is initially based on the medial and lateral defect localization in the abdominal wall [7]. The defect localization of medial incisional hernias is then further specified with the terms subxiphoidal, epigastric, umbilical, infraumbilical, and suprapubic. For lateral defects, a distinction is made between subcostal, lateral, iliac, and lumbar. Since the defect width has an unfavorable influence on the postoperative outcome in the treatment of incisional hernias, it is particularly considered in the classification. Depending on the defect width, incisional hernias vary in W1 (< 4 cm), W2 (≥ 4–10 cm), and W3 (> 10 cm) [7]. If multiple hernia defects ("Swiss-cheese" hernia) exist, they are combined when measuring the length and width of the defect. Due to poorer outcomes, recurrent incisional hernias, which account for approximately 25% of the total incidence of incisional hernias, are classified separately [7, 9, 10].
Diagnostics
In the diagnosis of abdominal wall hernias, in addition to clinical examinations, ultrasound, magnetic resonance imaging (MRI), and computed tomography (CT) are used [11]. A systematic review of incisional hernia diagnostics concludes that the prevalence of incisional hernia increases when other diagnostic methods are applied compared to purely clinical examination [13]. When comparing the different diagnostic methods, CT examination provides the most reliable results [11].
Abdominal wall hernias with defect widths of more than 10 cm are considered complex hernias [12]. Preoperative CT or MRI examination is increasingly required for these patients to plan the surgical strategy and risk assessment, as component separation is usually necessary, and patients must be informed preoperatively about its increased complication rate [13, 15]. CT or MRI examination also provides information about the condition of the abdominal wall musculature, which is crucial for planning the appropriate reconstruction method [13].
Tailored Approach
For the treatment of abdominal wall hernias with a defect size of ≥ 2 cm, a mesh technique is recommended [2]. The guidelines of the European Hernia Society and the American Hernia Society indicate that the use of a mesh is already indicated for epigastric and umbilical hernias with a diameter of ≥ 1 cm [3]. A suture technique should only be used for defects of < 1 cm [3].
Due to the different recommendations, there is a margin of discretion for defects of 1 to 2 cm. For primary abdominal wall hernias, a mesh technique should be used at least for a defect diameter of over 2 cm [2, 3]. A preperitoneal mesh technique is recommended in the guidelines of the American Hernia Society and the European Hernia Society for defects up to 4 cm, but there is also the option to treat the defect using the minimally invasive sublay techniques E/MILOS, eTEP, or TES.
The aforementioned recommendations primarily apply to patients without obesity (Body Mass Index [BMI] < 30 kg/m²) and/or rectus diastasis as well as defects up to 4 cm in diameter. Recent studies show that in obesity, laparoscopic IPOM has a lower wound complication rate compared to open procedures for abdominal wall hernias [5, 6]. Therefore, minimally invasive techniques should primarily be used in obesity [5, 6]. New techniques such as E/MILOS, eTEP, and TES can be prioritized to avoid the risks of intra-abdominal mesh placement, although there are no comparative studies yet.
In primary abdominal wall hernia with concurrent rectus diastasis, preperitoneal mesh technique and laparoscopic IPOM are insufficient [4, 5, 6]. Here, the new minimally invasive techniques, where the mesh is placed in the sublay layer, are suitable [4, 5, 6]. Alternatively, the medial portions of the two anterior layers of the rectus sheaths can be used to anatomically reconstruct the linea alba and close the defect. In the Endoscopic-assisted Linea-alba Reconstruction (ELAR), the mesh is used solely for augmentation [14].
For defects > 4 cm, the approach should be similar to that for incisional hernias. After lateral, combined lateral and medial incisions, and after previous incisional hernia repairs with meshes, sublay techniques are usually not possible due to significant scar changes in the extraperitoneal/retromuscular layer.
Possible Risk Markers for Unfavorable Outcomes
An analysis shows that suture techniques for umbilical hernias with a diameter of less than 2 cm have a significantly higher recurrence rate compared to mesh techniques [15]. Female gender is also associated with a higher risk of recurrence [15]. However, suture techniques have lower postoperative complications. Although laparoscopic IPOM has a lower risk of postoperative complications for small (< 2 cm) umbilical hernias, intraoperative complications, recurrences, chronic pain, and general complications are more frequent [15].
For umbilical hernia with larger defect widths and open procedures, more postoperative complications, complication-related reoperations, and general complications must be expected [16]. The risk of rest and exertion-dependent pain as well as chronic pain requiring treatment in the 1-year follow-up is increased in women and in the presence of preoperative pain [16, 17]. Increased defect width, increased BMI, and lateral defect localization lead to significantly more recurrences [16].
Technical Aspects
1. Surgical Techniques for Primary Abdominal Wall Hernias
1.1 Suture Technique
For performing the suture technique in umbilical and epigastric hernias with defects of less than one centimeter, quickly absorbable suture material is not recommended. However, there is limited evidence in the literature for slowly absorbable and non-absorbable suture material [3]. Both continuous and interrupted suture techniques can be used. The defect closure should be performed edge-to-edge.
1.2 Preperitoneal Mesh Technique
According to the guidelines of the European Hernia Society and the American Hernia Society, the preperitoneal mesh technique is recommended for umbilical and epigastric hernias with defects of ≥ 1–4 cm without rectus diastasis [3]. Defect enlargement to simplify mesh placement should be avoided. To prevent direct contact of the abdominal viscera with the mesh, parts of the hernia sac are used to close the peritoneal gap. The non-absorbable mesh (usually round) should overlap the defect by 3 cm on all sides, and the peritoneum around the defect must be dissected from the abdominal wall on all sides. The fixation of the mesh is done using non-absorbable sutures. Then, the fascial defect above the mesh is closed with slowly or non-absorbable sutures.
2. Surgical Techniques for Primary and Secondary Abdominal Wall Hernias
2.1 Laparoscopic Intraperitoneal Onlay Mesh Technique
According to the guidelines of the European Hernia Society and the American Hernia Society, the laparoscopic IPOM technique is recommended for larger primary abdominal wall hernias and in patients with an increased risk of wound complications [3]. This particularly applies to patients with obesity (Body Mass Index ≥ 30 kg/m²) and patients with a defect size of over 4 cm [3]. For incisional hernias, the defect should not exceed 8-10 cm [5, 6]. When using the laparoscopic IPOM technique, a defect closure should always be aimed for [3, 5, 6]. This reduces the seroma rate, avoids a remaining bulge of the abdominal wall, and minimizes recurrences [3, 5, 6]. The mesh must overlap the defect by at least 5 cm on all sides [3, 5, 6]. The fixation of the mesh is done using staples and sutures [3, 5, 6].
2.2 Minimally Invasive Sublay Techniques E/MILOS, eTEP, and TES
The minimally invasive sublay techniques offer an alternative to the preperitoneal mesh technique and laparoscopic IPOM for primary abdominal wall hernias, especially in conjunction with obesity and/or rectus diastasis. For incisional hernias, they provide an alternative to open sublay surgery and laparoscopic IPOM when defects of 8-10 cm are present.
2.2.1 E/MILOS (endoscopic mini/less open sublay)
The E/MILOS operation is a minimally invasive hybrid procedure that allows the placement of large synthetic meshes in the medial and lateral abdominal wall. The operation begins in a mini-open technique with incisions of up to 5 cm ("mini-open"), possibly 6-12 cm ("less open") [18, 19]. The preparation is then performed transhernially either with light-armored laparoscopic instruments under direct vision or with gasless endoscopy. After the use of a monopore or a gas-tight blockable optical trocar, the MILOS operation can be performed endoscopically [18, 19].
After classic preparation of the hernia sac and the fascial edges, the hernia sac is opened, the contents identified, repositioned, or resected. Intra-abdominal adhesions can be resolved through the hernia defect either openly or laparoscopically. After possible resection of excess hernia sac parts, the peritoneum is closed, and the strictly extraperitoneal blunt preparation of the retromuscular layer is performed. With complete preparation of the medial compartment, standard synthetic meshes up to a maximum size of approximately 40 × 20 cm can be placed flat on the mesh bed. Due to the defect overlap of the mesh by at least 5 cm, fixation can usually be omitted [4]. Posterior component separation is also possible with the MILOS operation. Giant hernias with "loss of domain" are not suitable for the MILOS technique.
2.2.2 eTEP ("extended totally extraperitoneal")
The eTEP technique is based on the experiences with total extraperitoneal patch plasty (TEP) in the treatment of inguinal hernias [20]. For hernias at and above the navel, the first access is chosen in the area of the right rectus sheath a few centimeters below the navel [20]. After displaying the anterior layer of the right rectus sheath, it is opened, the rectus muscle is lateralized, and then a balloon trocar is introduced extraperitoneally and an appropriate space is dilated. After the introduction of a gas-tight optical trocar and insufflation of CO2 gas, two working trocars can then be placed above the symphysis under vision. Subsequently, a change in the working direction occurs, in which both posterior layers of the rectus sheaths are detached at the medial edge, connecting both retrorectal spaces [20].
In the area of the navel or proximal to it, the hernia sac is encountered. It is opened at the edge, the contents are repositioned or resected. Then, the two posterior layers of the rectus sheaths are detached medially above the hernia gap up to the xiphoid. By closing the defect in the area of the hernia with sutures, the same retrorectal space is created as in the E/MILOS operation. On the mesh bed consisting of the posterior layers of the rectus sheath, the peritoneum, and the fascia transversalis, a sufficiently large mesh can then be placed [20]. There are various modifications regarding the access points or positioning of the trocars for the eTEP technique [21].
2.2.3 TES (Totally-endoscopic-Sublay-Technique)
The TES technique differs from the eTEP method only in that the extraperitoneal space is not created by an optical trocar in the right rectus sheath below the navel, but by an optical trocar directly above the symphysis and by creating the extraperitoneal space through blunt preparation. Once the extraperitoneal space is opened, two working trocars can be introduced under vision [22]. The further steps of preparation and mesh insertion are then carried out according to the eTEP method [22].
3. ELAR (Endoscopic-assisted Linea-alba Reconstruction)
Especially in young, slender women after childbirth, endoscopic-assisted linea-alba reconstruction can be considered a therapeutic alternative for the treatment of primary abdominal wall hernias with concurrent rectus diastasis [4, 8, 14, 23].
In this method, a curved skin incision is made with circumcision of the navel, which is continued up to 2 cm proximally in the midline. Similar to the E/MILOS operation for the repair of symptomatic umbilical and epigastric hernias, the classic treatment of the hernia sac is then performed. After dissecting the navel from the abdominal wall fascia, the medial part of the two anterior layers of the rectus sheath is dissected in the area of the rectus diastasis. The preparation is carried out proximally and distally from the navel until the distance between the two rectus muscles is only 2 cm, which may be necessary up to the xiphoid and far below the navel, depending on the findings. Preparation beyond the skin incision requires the use of a videoendoscopic camera with a light source.
Subsequently, the anterior layer of the rectus sheaths is incised about 1-2 cm from the medial edge, and the two medial portions of the anterior layers of the rectus sheaths are adapted in the midline with a continuous, non-absorbable suture. This creates a new linea alba, the defects are closed, and the rectus musculature returns to the midline. For complete anatomical reconstruction, a mesh is sewn into the defect as a replacement for the anterior layer of the rectus sheaths. This method is considered the simplest technique of component separation and is also performed as "myofascial release" in plastic surgery without additional mesh reinforcement [14, 23].
4. Surgical Techniques for Secondary Abdominal Wall Hernias
So far, the laparoscopic IPOM and the sublay technique have been the usual surgical techniques for a simple incisional hernia with a defect width of < 8–10 cm [5,6]. The laparoscopic IPOM is increasingly being replaced by minimally invasive sublay techniques due to the potential dangers of intra-abdominal mesh placement [24]. This led to the development of the "tailored retromuscular approach," meaning that the various retromuscular techniques (E/MILOS, eTEP, and TES, sublay, posterior component separation/transversus-abdominis-release) are used depending on defect width and individual patient characteristics.
The basic techniques of E/MILOS, eTEP, TES, and laparoscopic IPOM have already been presented for primary abdominal wall hernias. It has already been demonstrated that incisional hernias can be efficiently treated using the E/MILOS technique compared to open sublay surgery and laparoscopic IPOM [19].
4.1 Open Sublay Technique
The sublay technique describes a retromuscular preperitoneal position of the mesh, ideally involving a midline reconstruction with closure of the fascia over the mesh. The mesh is implanted under the rectus abdominis muscle on the posterior layer of the rectus sheath and the fascia transversalis [24]. A good mesh bed with sufficient blood supply and a lower infection risk are the reasons for the advantages of this technique. The intra-abdominal pressure rests on the mesh as the strongest component of the closure and supports its fixation. This can achieve a low recurrence rate [25, 26].
4.2 Posterior Component Separation/Transversus-abdominis-Release
The initial steps of the transversus-abdominis-release are similar to the sublay technique. Therefore, the sublay operation can be intraoperatively extended to the transversus-abdominis-release if the detachment of the posterior layers of the rectus sheaths is insufficient for a defect closure due to a lack of sufficiently large mesh bed for larger defects. Differential therapeutic considerations must take into account the transversus-abdominis-release for incisional hernias in the midline with a defect width of ≥ 10 cm. A preoperative computed tomography is very useful in this regard.
With the detachment of the two posterior layers of the rectus sheath from the xiphoid down to the linea arcuata, the sublay part of the transversus-abdominis-release is completed. The space between the xiphoid and "fatty triangle" is opened cranially, and the preperitoneal space between the rectus muscles and fascia transversalis/peritoneum or symphysis and bladder is accessible caudally. This provides an extraperitoneal mesh bed on which meshes of 30 x 30 cm and larger can be placed.
According to initial publications, it is also possible to apply the open transversus-abdominis-release technique as an addition to eTEP, E/Milos, or robot-assisted [3, 27].
4.3 Open IPOM
An extraperitoneal or retromuscular mesh placement is usually not possible for incisional hernias after transverse, lateral, and combined medial-lateral incisions, as well as for recurrences due to pronounced scarring [27]. In these cases, the open IPOM technique is the only therapeutic option [27]. Compared to the sublay technique, the open IPOM technique leads to more chronic pain in the 1-year follow-up [28]. There is a considerable difference in the postoperative complication and recurrence rates for open IPOM in the literature. A wide overlap of the mesh, avoidance of dissection in the abdominal wall, and defect closure are required to achieve better results with open IPOM [27]. It is also possible to use parts of the hernia sac as a defect closure [27].
4.4 Open Onlay
A study found that the onlay technique has a comparatively high rate of postoperative complications compared to the sublay technique [29]. An investigation of the Herniamed registry showed that there are no significant differences in the results for small and lateral incisional hernias between sublay and onlay techniques [30].
Sufficient overlap of the mesh (at least 5 cm) and defect closure also lead to more favorable results with open onlay. It is also possible to use a doubled hernia sac for defect closure. Postoperatively, drainage and possibly abdominal binders should be used, as seromas are more common with open onlay [29].
Robot-assisted Surgery for Ventral Abdominal Wall Hernias
The development of robot-assisted retromuscular procedures has enabled significant advancements in the surgery of ventral abdominal wall hernias. With the availability of robots, procedures with retromuscular mesh placement can now be performed entirely minimally invasively.
In 2018, Muysoms et al. and Belyansky et al. described robotic modifications of minimally invasive surgical procedures with retromuscular mesh reinforcement for a fully minimally invasive treatment of abdominal wall hernias [31, 32]. While Muysoms described a robot-assisted transabdominal repair of umbilical hernias with retromuscular technique (robotic-transabdominal retromuscular umbilical patch plasty, r-TARUP), Belyansky favored an extraperitoneal approach for a robotic "enhanced view totally extraperitoneal plasty" (enhanced view totally extraperitoneal plasty, r-eTEP). With the help of robotics, the transversus-abdominis-release established in open surgery is also possible minimally invasively [33].
So far, only completed randomized controlled trials with long-term follow-up are available for robotic IPOM mesh positioning. In a multicenter study by Dhanani et al., 124 patients were randomized, of whom 101 completed the 2-year follow-up [34]. While no significant differences in the perioperative course were found in the first publication, the analysis of the 2-year results now shows initial advantages of the robotic technique with a lower recurrence rate and a significantly lower reoperation rate [34, 35]. For retromuscular procedures, only systematic reviews and meta-analyses on the perioperative course are currently available, and studies with longer follow-up are lacking.
In 2021, Bracale et al. published a meta-analysis that included studies published up to September 2020 comparing open and robotic transversus-abdominis-release [36]. In this systematic review, the robotic group showed a significantly lower overall complication rate, a significantly shorter hospital stay, and a significantly longer operation duration. A statistically significant difference in wound infection rate was not found.
In 2022, Dewulf et al. published a case observation study from two European hernia centers comparing the early postoperative outcomes of a total of 90 robotic and 79 open transversus-abdominis-release operations [37]. It was found that the duration of the postoperative hospital stay was significantly shorter in the robotic group (3.4 vs. 6.9 days). In the open group, serious complications (20.3% vs. 7.8%) and wound complications occurred significantly more frequently (12.7% vs. 3.3%).