Perihilar/central bile duct carcinoma (Klatskin tumor) originates from the bifurcation of the extrahepatic bile ducts. Due to the almost absent muscular layer of the bile ducts, there is early invasion into the liver parenchyma or the portal vein.
The only curative treatment option is radical R0 resection, which can only be achieved through an en bloc resection of the bile ducts, bile duct bifurcation with liver resection. The assessment of resectability and development of an appropriate resection strategy is of utmost importance due to the close anatomical relationship to the portal vein bifurcation and the hepatic arteries.
Curative approaches in advanced stages are regularly associated with significant loss of liver parenchyma and pose a challenge for surgery, anesthesia, and intensive care medicine.
An isolated resection of the extrahepatic bile duct system (so-called "isolated hilus resection") cannot be considered a curative procedure. The result is local recurrence rates of 70-90% and virtually no long-term survival. The bile ducts of the caudate lobe (Segment 1), which regularly open near the bile duct bifurcation, are predisposed to local recurrences.
To achieve an adequate safety margin, a simultaneous en bloc resection of the central liver parts together with the bile duct system is always necessary. This includes the caudate lobe as well as the hilar portions of segments 4, 5, and 8. Achieving a proximal and lateral safety margin is often problematic due to the usually diffuse infiltration along the bile ducts and perineural sheaths.
Extended hemihepatectomies have been established as standard procedures, with right-sided procedures requiring the so-called hilar en bloc resection including the portal vein bifurcation. In left-sided procedures, due to the course of the right hepatic artery, dissection is usually required dorsal to the bile duct, so a general portal vein resection is not indicated here.
In an inoperable situation due to technical reasons or functional irresectability, liver transplantation is an option, although with poor results so far and a glaring shortage of organs. Improved results are available for selected patients in a multimodal treatment program.
For these extensive liver resections of an organ damaged by cholestasis and bile duct inflammation, preoperative assessment of residual liver function is essential. Conditioning of the liver through selective bile duct drainage and, before extensive right resections, through hypertrophy induction (portal vein embolization/ALPPS) is an option for improved outcomes.
Especially when hypertrophy induction is not possible, the procedure described here of central liver resection is a technical variant for radical resection while simultaneously maximizing the preservation of healthy liver parenchyma.
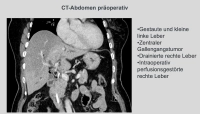
In the demonstrated case, it is a stenosing bile duct tumor at the hepatic bifurcation Bismuth IIIb, diagnosed by MRCP, ERCP, and brush cytology. An extended right resection was not feasible due to atrophy of the left liver lobe. Therefore, removal of liver segments 1, 4, 5, and 8 was performed while preserving the lateral segments 6, 7, and 2, 3, particularly since the portal vein bifurcation and the left hepatic artery did not appear infiltrated morphologically.