Medical History
- local symptoms of thyroid enlargement, nodule growth (cervical pressure or globus sensation, swallowing difficulties, and dyspnea, especially under exertion)
- voice changes/hoarseness, recurrent laryngeal nerve palsy
- symptoms of hyperthyroidism
- medications (iodine-containing preparations, antithyroid drugs)
- family medical history
- previous radiation in the neck area: A confirmed etiological factor for the development of differentiated thyroid carcinoma is exposure to ionizing radiation, which is why patients with thyroid nodules should be specifically asked about previous radiation to the head-neck region.
- Pre-existing cervical spine problems (head reclination during positioning!)
Physical Examination
- palpation (size, consistency of thyroid lobes, nodules, swallowing mobility, palpable lymph nodes)
- endocrine ophthalmopathy
Vocal Cord Function Testing
- A preoperative ENT laryngoscopy to assess vocal cord mobility is essential.
- It can detect pre-existing damage to the recurrent laryngeal nerve, e.g., after previous surgery or in malignancy.
- It allows for a situation-adapted surgical strategy.
- It is the basis of perioperative quality assurance
- Pre- and postoperative laryngoscopy and intraoperative neuromonitoring form the basis of perioperative quality assurance and are an inseparable diagnostic unit. Neuromonitoring is not usable without knowledge of clinical laryngeal function! LINK IONM
Laboratory Tests
- usual preoperative laboratory parameters depending on underlying disease, coagulation, calcium, PTH
- TSH, thyroid hormones: fT3, fT4
The most important in-vitro parameter is TSH, whose pathological change is based on a long-standing thyroid dysfunction. Note: A low concentration suggests hyperthyroidism, a high concentration suggests hypothyroidism. In these cases, additional determination of thyroid hormones (fT3 and fT4) is mandatory, while with normal TSH and clinical euthyroidism, it can be omitted.
- thyroid antibodies for the diagnosis of immunothyropathy and thyroiditis
- Antibodies against TSH receptor (TRAK) should be determined if a clear distinction between Graves' disease and non-immunogenic hyperthyroidism is not possible based on clinical examination and imaging.
- Antibodies against thyroid peroxidase (anti-TPO) are determined when autoimmune thyroid disease is suspected; they are elevated in 95% of patients with Hashimoto's thyroiditis (autoimmune thyroiditis) and in 70% of patients with Graves' disease.
- Antibodies against thyroglobulin (anti-TG) are less specific, determined additionally when autoimmune thyroiditis is suspected, especially if anti-TPO is negative.
- Determination of basal calcitonin level.
Calcitonin (Ctn) is a highly specific tumor marker for medullary thyroid carcinoma (MTC). German guidelines recommend a one-time determination before any thyroid surgery to detect C-cell changes in early form. The level of basal calcitonin allows conclusions about the stage of the disease and helps plan the extent of resection.
From a threshold of ≥ 30 pg/ml Ctn for women and ≥ 60 pg/ml for men, an MTC is likely enough to justify further treatment steps.
Lymph node metastases in the lateral lymph node compartment seem to occur only at a calcitonin level above 85/100 pg/ml and simultaneous evidence of desmoplasia. Otherwise, lateral lymph node dissection can be omitted. 25% of tumors are familial, e.g., leading tumor in MEN 2a. Therefore, molecular genetic clarification is always recommended in suspected MTC.
- Note: Smoking, proton pump inhibitors, renal insufficiency, and chronic alcohol consumption can lead to slightly to moderately elevated calcitonin levels (control under abstinence if necessary).
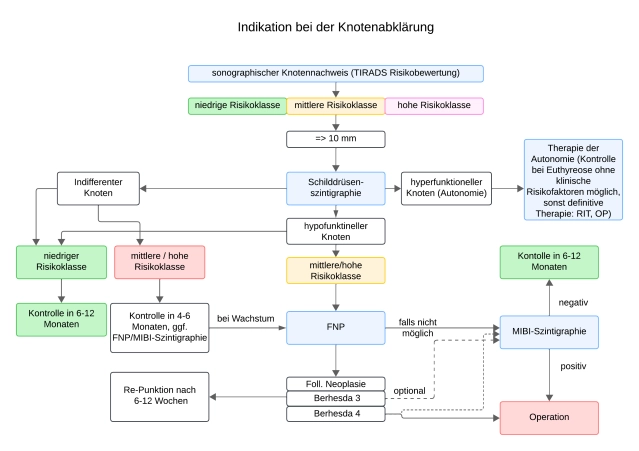
Ultrasound with TI-RADS Classification (Thyroid Imaging Reporting and Data System)
Preoperative neck ultrasound is of great importance in surgical planning. It is the basic examination method for assessing thyroid morphology.
The standardization of thyroid nodule findings allows for an assessment of dignity or risk stratification of the nodule, as required by guidelines.
Nodular findings of the thyroid are described in detail under documentation of the following criteria:
- Size (specify diameter in all 3 planes)
- Echogenicity (hypoechoic, isoechoic, hyperechoic, anechoic, and complex echo)
- cystic components
- micro- or macrocalcifications
- presence of a hypoechoic rim (halo sign)
- margin delineation (sharp versus blurred)
- configuration (asymmetric, "taller than wide")
- vascularization
The following sonographic criteria are associated with a significantly increased likelihood of malignancy:
- Hypoechogenicity
- blurred margins
- non-oval shape
- "Taller-than-wide" shape: nodule is more pronounced in depth than in width in the axial section.
- Presence of microcalcifications
LINK TIRADS
Note:
Ultrasound can also assess the relationship to neighboring structures, lymph node status, and possible retrosternal extension.
Thyroid Scintigraphy
Scintigraphy with the tracer 99m-Technetium pertechnetate (Tc-99m) has a discrimination limit of about 1cm for lesions that are either more, equally, or less storing than the surrounding tissue and are thus described as scintigraphically warm/hot (with simultaneous suppression of surrounding tissue), indifferent, or cold.
Notes:
- The scintigraphy, together with ultrasound, is the basic examination method in the evaluation of thyroid nodules.
- Autonomous areas that are no longer subject to regulatory control by TSH can be unmasked by suppression scintigraphy (with orally administered thyroxine).
- Even with a normal TSH value, functionally autonomous nodules can be present. These should not be punctured.
- Scintigraphically cold nodules that are sonographically anechoic correspond to cysts and are considered benign.
- Non-anechoic cold nodules require clarification
Optional Preoperative Diagnostics
Magnetic Resonance Imaging/Native Computed Tomography
- In retrosternal goiter, to assess the extent of the retrosternal portion, facilitating preoperative planning of a possibly necessary thoracic surgical access route (sternotomy).
- in pronounced local compression symptoms
- in organ-overlapping growth
Note 1: These two examinations have no role in the initial assessment of thyroid nodules.
Note 2: In differentiated thyroid carcinoma (DTC), MRI should be preferred to avoid contrast exposure.
Computed tomography has the disadvantage that contrast with contrast medium must be avoided, partly due to the risk of iodine-induced hyperthyroidism, and partly to avoid iodine contamination in anticipation of radioiodine therapy. After exogenous iodine intake, iodine receptors are blocked for a longer period, making radioiodine therapy and thyroid scintigraphy impossible.
PET-CT
- Molecular whole-body imaging in advanced tumors for recurrence and metastasis diagnostics with functional tracers such as dopamine (18F-DOPA), somatostatin analogs (68Ga-DOTATOC) in MTC, and fluorodeoxyglucose (18F-FDG) in PDTC (Poorly Differentiated Thyroid Carcinoma), which often does not store radioiodine.
Scintigraphy with ⁹⁹ᵐTc-MIBI with Washout Index as a Semi-Quantitative Method
For the assessment of the dignity of thyroid nodules, sestamibi scintigraphy is used clinically off-label. Off-label use must be disclosed. MIBI scintigraphy should only be used in scintigraphically hypofunctional nodules from 1 cm in size and suspicious sonomorphology (TIRADS 4 and 5) and evidence of a follicular neoplasm (Bethesda 3/4).
Imaging with ⁹⁹ᵐTc-MIBI can be used especially when fine needle aspiration is indicated but not possible or has not yielded conclusive results.
The predictive negative predictive value with rapid washout is good, follow-up controls are required.
Fine Needle Aspiration Cytology
Based on clinical, sonographic, and scintigraphic criteria, risk nodules are identified, which are then further clarified by fine needle aspiration. Since multinodular thyroid changes are common in Germany, it is advisable to limit the need for aspiration to non-autonomous nodule areas >1 cm – depending on sonographic features.
Fine needle aspiration (FNA) of a suspicious thyroid nodule serves to assess the risk of malignancy. It is particularly indicated when non-surgical treatment of the lesion is considered.
In the following scenarios, an indication for aspiration of thyroid nodules may arise:
- Patients with clinical signs of thyroid carcinoma when cytological diagnosis is important for surgical planning.
- Nodules depending on size from EU-TIRADS classification class 3: class 3 (> 2 cm), class 4 (> 1.5 cm), class 5 (> 1 cm)
- Nodules of any size with extracapsular growth or unclear cervical lymph nodes (here, the lymph node should also be aspirated if necessary)
- Nodules of any size in patients with a history of neck radiation without evidence of autonomy
- First-degree relative of a patient with papillary or medullary thyroid carcinoma or multiple endocrine neoplasia type 2
Note: In MTC, Ctn determination is superior to cytology. Due to the special importance of calcitonin screening, FNA is generally not required for the preoperative diagnosis of MTC. The assessment of a desmoplastic stromal reaction in histology is no longer reliably possible after FNA, so FNA should not be performed with elevated Ctn.
Aspiration should be explicitly avoided in nodules that correspond to scintigraphically focal autonomies, as well as in nodules that do not exhibit any sonographic criteria suspicious for malignancy.
Cytologically, many tumor entities can be diagnosed with high accuracy. Follicular neoplasia requires histological clarification.
The indication for surgery is based on cytology in cases of follicular neoplasia, detection of specific mutations, or other evidence or proof of malignancy.
Detection of benign findings in FNA can avoid unnecessary surgeries. Detection of malignant cells significantly influences the surgical strategy (hemithyroidectomy versus thyroidectomy, extent of lymph node dissection).
In encapsulated follicular tumors, differentiation between follicular adenoma and carcinoma is not possible by FNA. Similarly, FNA cannot distinguish between a non-invasive encapsulated follicular variant of papillary thyroid tumor (NIFTP) and papillary thyroid carcinoma. Molecular pathological additional investigations can increase the sensitivity and specificity of FNA but are not yet routinely used.
The most common papillary carcinoma, accounting for over 80% of all differentiated thyroid carcinomas, can be diagnosed with high reliability.
Cytopathological Evaluation of FNA
The Bethesda classification is a 6-grade system designed exclusively for thyroid aspiration and is largely evidence-based. The advantage of this system lies in the clear assignment of malignancy probability in the individual groups. Moreover, this classification allows for international comparability due to its international use.
LINK Bethesda Classification
In suspected transmural infiltration, additionally panendoscopy, tracheoscopy, and esophagoscopy